Transforming diagnosis and treatment planning through advanced gene expression profiling
SKY92 is a standardized assay based on 92 genes extracted from malignant plasma cells to assess the myeloma’s aggressiveness at diagnosis. Developed on a microarray platform, SKY92 is a CE-IVD marked kit in Europe and CLIA validated Lab Developed test in the United States.
The outcome of the test is binary:
Standard-risk
the myeloma is less aggressive; these patients have a favorable progression free and overall survival.
High-risk
the myeloma is aggressive; these patients have a worse progression free and overall survival.
Developed on a microarray platform, SKY92 is a CE-IVD marked kit in Europe and CLIA validated Lab Developed Test in the United States.

Bone marrow aspirate collected in EDTA or herpin.
Minimum 200.000 plasma cells (CD138 positive) isolated within 32h from BM aspirate.
Minimum of 200ng of total RNA.
MMprofiler Analysis
Physician / Patient Review Report
Analytical validation of the SKY92 assay was performed with primary bone marrow specimens of Multple Myeloma patients and cell line specimens in 3 independent laboratories.
Peer-reviewed manuscript detailing analytical validation; including reported assay success rate of 92%. Read more here.
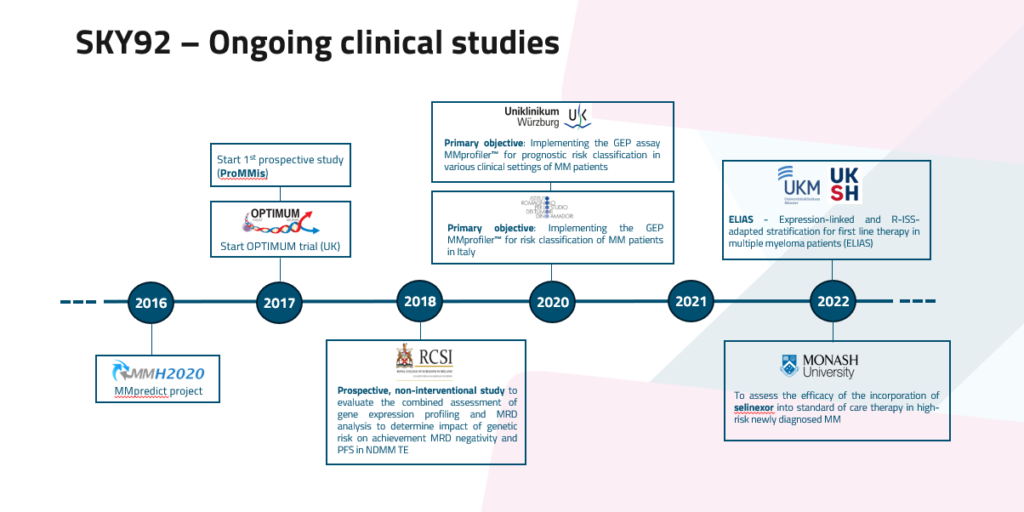
SKY92 has the most extensive validation across both trial and real-world cohorts and categorizes around 20-25% of NDMM as high-risk (Rees et al, 2024).

Contact our team to set up test orders:
Discuss SKY92 with your clinician to see if it’s right for you.
Yes, every submission that we receive will be evaluated by our team.
We will contact you initially within one working day. For the proposal, the review depends on the length and details, we will communicate with you how soon we can discuss it.
Newest publications
An independent Danish validation study has confirmed the Merlin Assay’s efficacy in predicting nodal metastases.
Findings in a combined US and EU multi-center validation study show the potential ability of CP-GEP to support in risk stratifying patients with T1a melanoma.
Findings in a combined US and EU multi-center validation study show the potential ability of CP-GEP to support in risk stratifying patients with T1a melanoma.
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), April 4, 2025—SkylineDx, an innovative diagnostics company specializing in molecular diagnostics for oncology, inflammatory, and infectious diseases, proudly announces that Dr. Teresa Amaral MD, PhD,...
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), March 28, 2025—SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology , inflammatory and infectious diseases, proudly announces...
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), March 12, 2025—SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology and, inflammatory and infectious diseases, proudly announces...
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), February 28, 2025—SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology, and inflammatory and infectious diseases, announces the...
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), December 7, 2024—SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology, inflammatory, and infectious diseases, announce that groundbreaking...
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), October 25, 2024—SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology, inflammatory, and infectious diseases, announced today the...