Transforming clinical research into biomarker discovery is the true foundational work and is instrumental to solving the clinical unmet needs in current care practice. We would appreciate collaborating with you and bringing your biomarker discovery to the patient. At SkylineDx, we bridge the gap between (academically) discovered biomarkers and commercially available molecular assays – taking your innovation from the bench to the bedside.
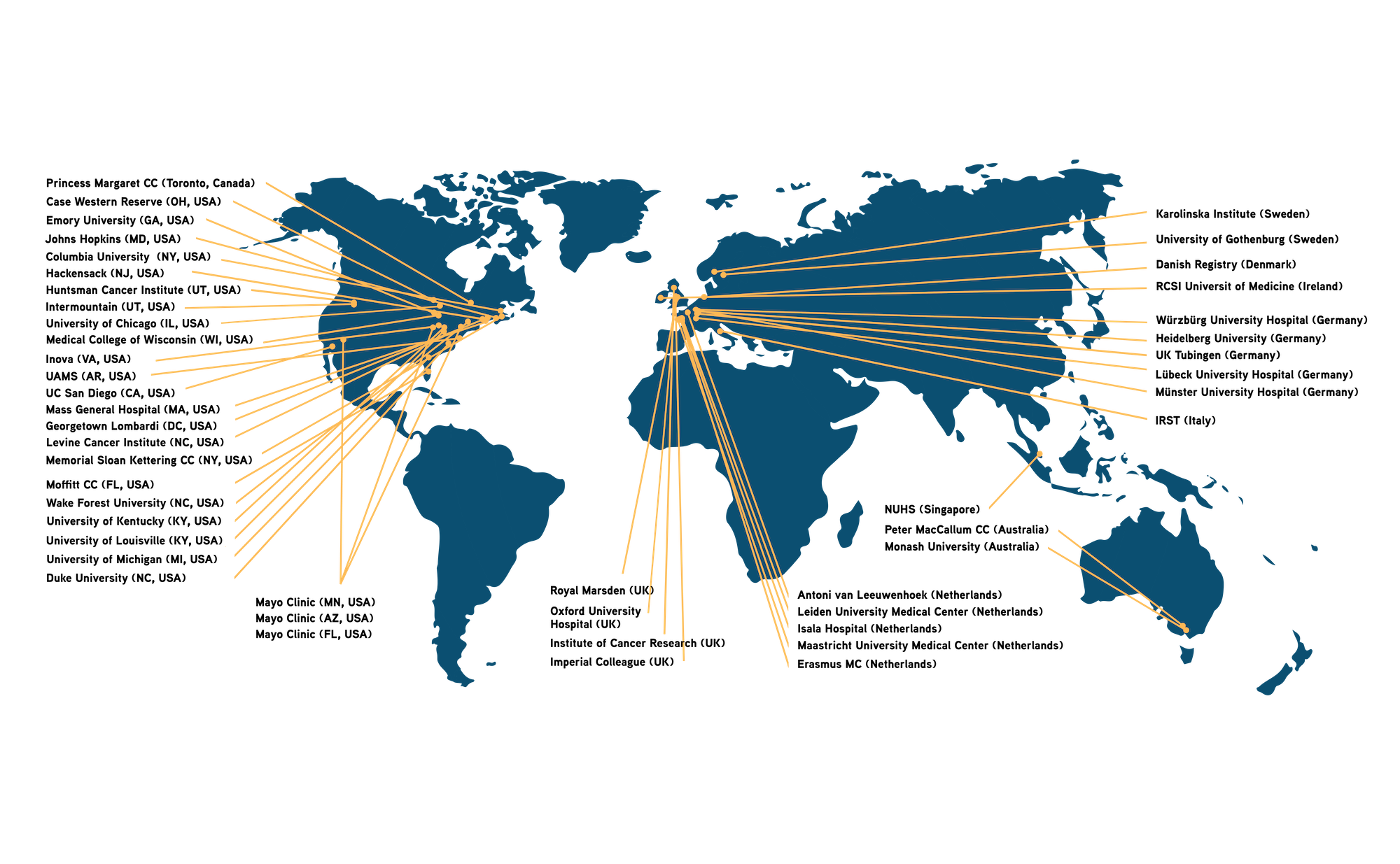
Our extensive network of researchers and academic institutions empowers us to identify the latest discoveries, technologies, and research insights to refine our diagnostic offerings.
We encourage you to submit your biomarker discovery or your proposal to write a grant application with us. We are always open to new collaborations. Whether regarding collaborations to collect additional evidence on products already in our pipeline or collaborations outside our current R&D Programs. We believe that scientifically robust and clinically meaningful biomarkers should find their way to patients, therefore we don’t focus on one disease or group of diseases specifically.
Before a new molecular biomarker enters our Research & Development Programs, all discoveries and research opportunities are thoroughly evaluated in our dedicated STRIX (Systematic, Translational R&D Workflow, building on Integrated Expertise) Program. Our interdisciplinary team searches, screens, and evaluates hundreds of high-potential biomarkers a year, selecting the most promising. We then partner with the inventor and potentially license the related intellectual property (IP) that allows us to develop and commercialize the biomarker.
We form strong partnerships with technology transfer offices from hospitals and academia, as well as close collaboration with the involved clinicians and researchers directly. When a biomarker progresses from STRIX to one of our R&D Programs, we might file and develop additional IP.
Strix is a genus of the owl family and symbolizes wisdom and perception. Its sharp perception of details, broad view, and ability to see in the dark closely resembles what the STRIX Program is capable of making visible which would otherwise remain hidden.
Melanoma is an aggressive type of skin cancer. In melanoma, we have an extensive collaboration named the Falcon R&D Program. As the falcon bird is known as an intelligent creature with unprecedented senses and skills, our R&D program is uniquely equipped to unveil new, detailed insights in the genomic, biologic and clinical nature of melanoma.
Under the wings of the Falcon R&D Program, a series of specific studies and projects has been initiated, aimed at developing and introducing an array of diagnostic utilities, to provide physicians with the tools to optimize the clinical pathway of their patients. Not by coincidence, related series of studies are named after birds of prey in the Falcon family.
The Panthera Program focuses on hematological malignancies, cancers that develop in blood cells. Tackling hematological cancer requires strength and intelligence. Conditions such as multiple myeloma hide deep in the bone marrow.
Infectious and Inflammatory diseases consist of many disorders and studies suggest that a dysfunction of the immune system seems to be an important shared characteristic. The first disease in this program is Kawasaki disease.
Yes, every submission that we receive will be evaluated by our team.
We will contact you initially within one working day. For the proposal, the review depends on the length and details, we will communicate with you how soon we can discuss it.
Newest publications
An independent Danish validation study has confirmed the Merlin Assay’s efficacy in predicting nodal metastases.
Findings in a combined US and EU multi-center validation study show the potential ability of CP-GEP to support in risk stratifying patients with T1a melanoma.
Findings in a combined US and EU multi-center validation study show the potential ability of CP-GEP to support in risk stratifying patients with T1a melanoma.

What others have to say about us







ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), April 4, 2025—SkylineDx, an innovative diagnostics company specializing in molecular diagnostics for oncology, inflammatory, and infectious diseases, proudly announces that Dr. Teresa Amaral MD, PhD,...
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), March 28, 2025—SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology , inflammatory and infectious diseases, proudly announces...
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), March 12, 2025—SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology and, inflammatory and infectious diseases, proudly announces...
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), February 28, 2025—SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology, and inflammatory and infectious diseases, announces the...
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), December 7, 2024—SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology, inflammatory, and infectious diseases, announce that groundbreaking...
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), October 25, 2024—SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology, inflammatory, and infectious diseases, announced today the...